One Engine. Two Tools. Built to Diagnose Differently
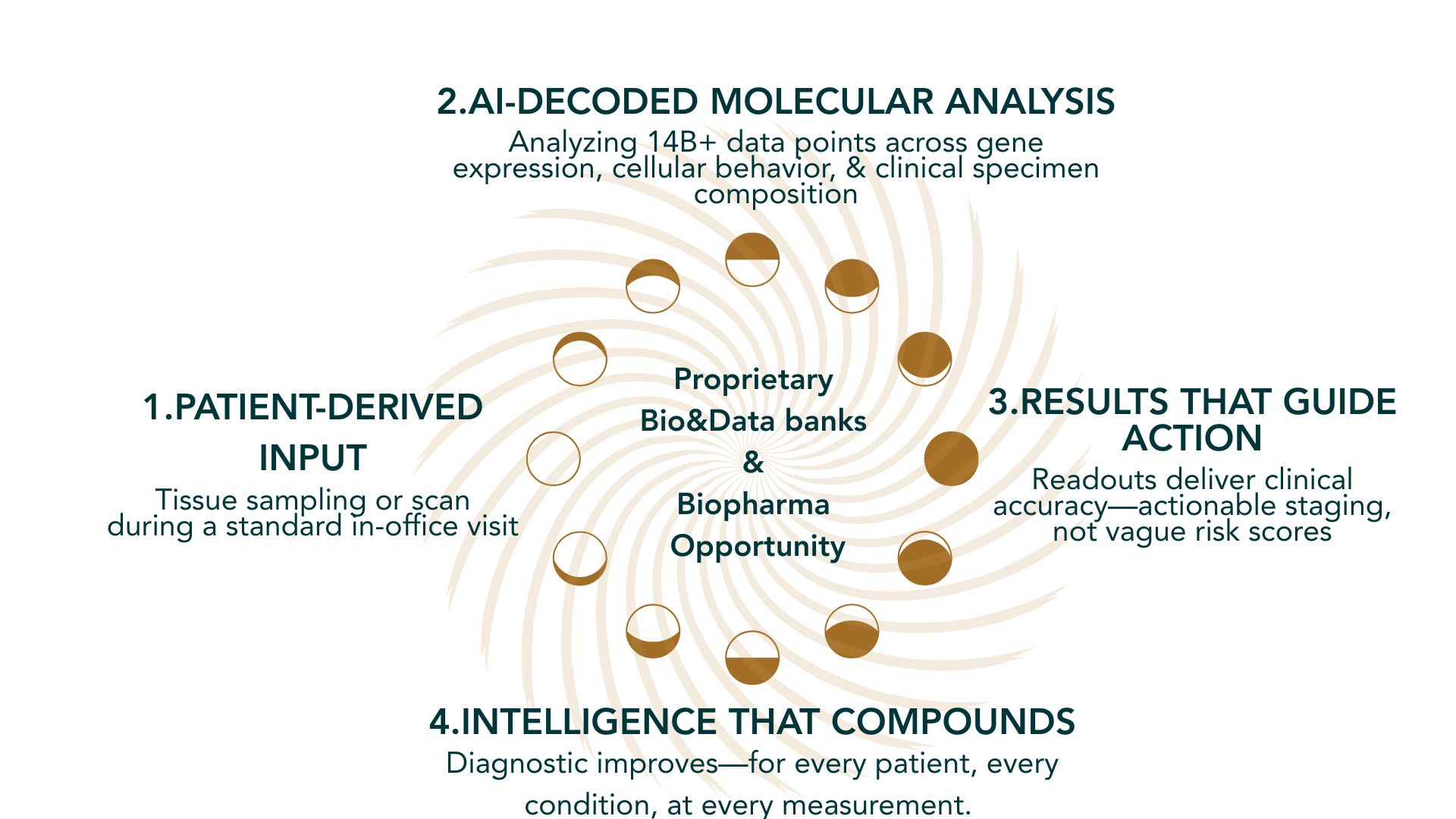
MetriDx™ and HERAfem™ aren’t two standalone products.
Two tools. One platform. Optimized for frontline care.
Each tool:
- Collects biological and physical information from the tissue
- Analyzes biological complexity using adaptive AI/ML models.
- Outputs are elaborated to improve each tool and develop new insights
Their differences don’t fragment the platform. Same learning engine. Shared advantage.
|
|
The First Non-Surgical Diagnostic That Confirms and Stages Endometriosis—From a Simple Office Visit |
|
A Real-Time Cervical Cancer Diagnostic That Detects CIN2+ Lesions—No Biopsy, No Lab, No Delay |
|---|---|---|---|
|
Sensitivity / Specificity |
80% / 95% |
|
91% / 65%* |
|
Diagnostic Target |
Detects & molecularly stages endometriosis |
|
Detects cervical intraepithelial neoplasia grade 2+ (CIN2+) lesions before they become invasive cancer |
|
Data Type |
mRNA expression profiling via RT-qPCR |
|
Optical & electrical spectroscopy |
|
AI Role |
ML model trained on 14B+ datapoints from gene expression |
|
On-device AI enables non-invasive classification |
|
Result Timing |
3-to-5 days via lab processing |
|
Same-visit, point-of care diagnosis |
|
Sample Collection |
Pipelle sampling—routine, non-surgical, no anesthesia |
|
Cervical scan - no biopsy, no cramping, no lab needed |
|
Care Setting |
OB-GYN, fertility clinics, primary care, reference labs |
|
OB-GYN, primary care, rural & underserved care environments |
|
Intellectual Property |
6 patents |
|
2 patents |
|
Regulatory Pathway |
LDT, FDA Approval |
|
COFEPRIS, FDA Approval |
|
Launch Timeline |
U.S. in 2026 |
|
Latin America in 2025 |
|
|
|
|
|
*Ongoing study to improve overall accuracy